Nipah Virus Outbreak Prevention and Control: BIOBASE Comprehensive Detection Solution for Nipah Virus
Recently, the Nipah Virus outbreak in India has attracted great attention from the public health sector. As a newly emerging infectious disease with high fatality rate and transmission risk, Nipah Virus imposes extremely high requirements on laboratory diagnostic capabilities, biosafety protection, and emergency response systems.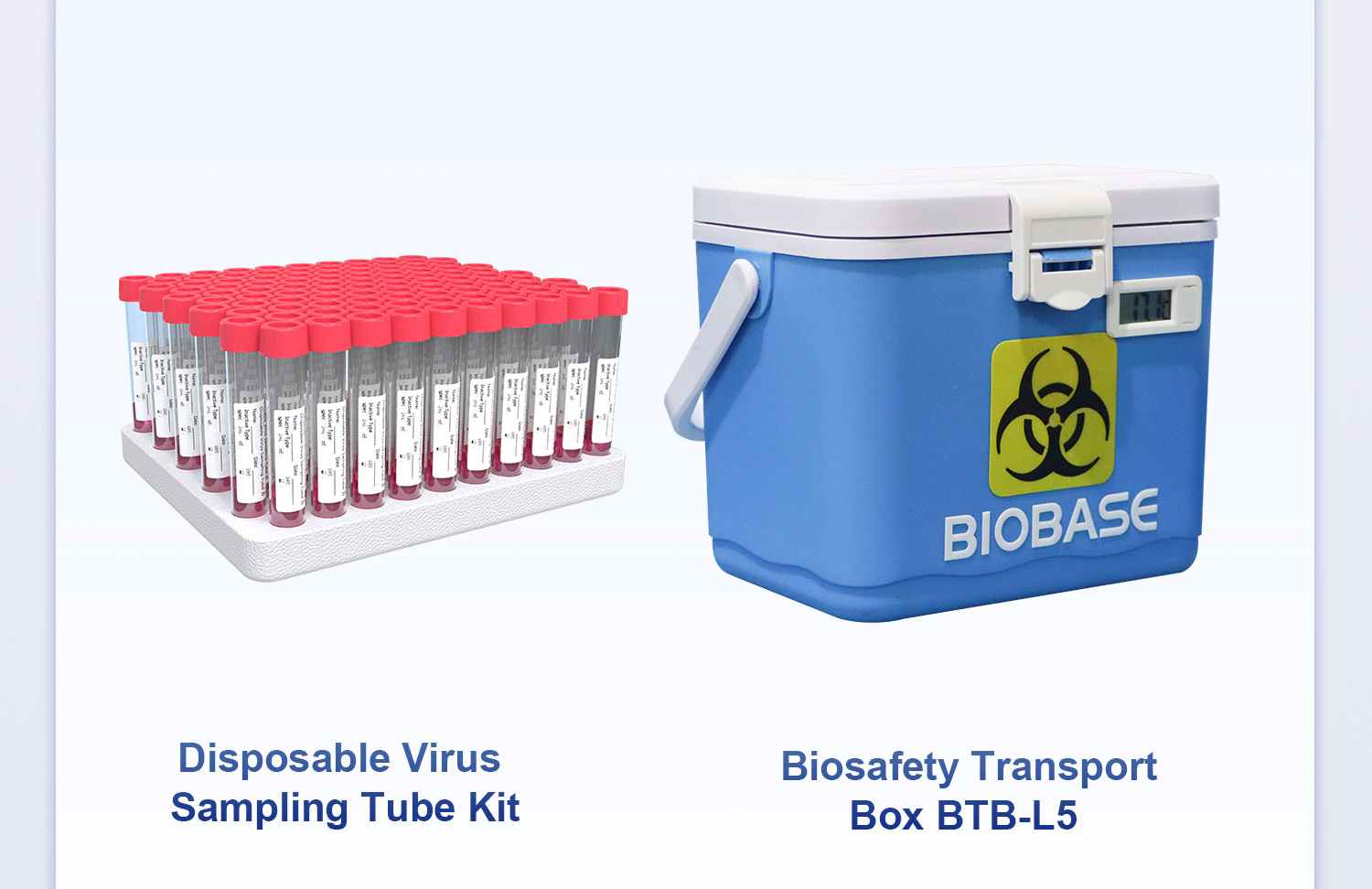
NO.1 Sampling and Transportation
In Nipah Virus testing, sampling and transportation are the first critical barriers for epidemic prevention and control. BIOBASE provides disposable virus sampling consumables and biosafety transport equipment, ensuring effective leak-proofing and contamination prevention during sample collection, sealing, and transportation, and minimizing biological risks to medical staff and the environment.
NO.2 Reagent storage and Preparation
The accuracy of test results relies on stable and reliable reagent management. BIOBASE offers laboratory refrigeration and low-temperature storage equipment for the standardized preservation of nucleic acid extraction reagents and related consumables. Meanwhile, it is equipped with clean operating environments and basic laboratory tools, providing low-contamination-risk operating conditions for reagent preparation and pre-processing.
NO.3 Sample extraction and storage
During the extraction, processing, and storage of samples involving high-risk pathogens such as Nipah virus, biosafety and sample integrity are critical. BIOBASE provides a comprehensive solution that integrates biosafety protection, automated processing, and ultra-low temperature storage, helping laboratories reduce operational risks, improve workflow efficiency, and ensure stable, reliable sample management throughout the entire testing process.
NO.4 Detection and result determination
During the nucleic acid testing stage for Nipah virus, BIOBASE’s molecular diagnostic detection systems support rapid and highly sensitive fluorescence quantitative analysis, enabling laboratories to obtain reliable results in a shorter time and providing critical support for epidemic surveillance and clinical decision-making.
NO.5 Waste disposal
Biohazard waste generated during infectious disease testing also requires strict management. BIOBASE's laboratory sterilization and disinfection equipment can standardize the treatment of pollutants, prevent secondary contamination, and build a complete biosafety closed loop.
Immune Detection Process
In addition to nucleic acid testing, immunoassays can serve as an important supplementary method in certain application scenarios. BIOBASE offers semi-automatic and fully automatic immunoassay solutions, suitable for different laboratory conditions, helping to improve testing throughput, reduce manual operation intensity, and meet continuous testing needs.
Virus Isolation and Identification (For Research/CDC Use Only)
In CDC and scientific research scenarios, BIOBASE provides equipment related to cell culture and microscopic observation, supporting virus isolation, identification, and basic research, and offering experimental conditions for in-depth understanding of virus characteristics and transmission mechanisms.
BIOBASE is committed to providing end-to-end laboratory solutions for global laboratories and public health institutions, covering sampling, testing, protection, and disposal, to support the response to challenges posed by high-risk infectious diseases such as Nipah Virus.
